Induction of the heat shock protein in rat lungs following intermittent hypoxic training
KoDo Okuyama 1), Jingtao Jiang 2)
Clinical Research Laboratory of ‘Mountain Air’ Therapy 2-21-15 Shimouma Setagaya-ku Tokyo JAPAN
Central Institute for Electron Microscopic Researches, Nippon Medical School, Sendagi, Bunkyo-ku, Tokyo, Japan.
Abstract
In order to demonstrate the mechanism of intermittent hypoxic training (IHT), we studied the expression of HSP70 by immunohistochemistry using the Streptavidin Biotin Peroxidase Complex(SABC) Method on alveolar type I , type II epithelial cells, macrophages from rats with IHT. The expression of HSP70 in IHT in comparison with control was significantly increased on alveolar type I , type II epithelial cells, macrophages and was significantly correlated with the duration of IHT.
This study demonstrates that expression of HSP70 may be a mechanism of adaptation of hypoxia by IHT.
Key words: Intermittent hypoxic training HSP70 Electron microcopy Immunohistochemistry
lntroduction
Intermittent hypoxic training (IHT), with repeated short-term inhalation of hypoxic mixtures, has been used to treat and prevent certain diseases and has a very favorable effect for the exercise of athletes 1,2. Investigations of IHT have showed that increased hypoxic ventilatory response (HVR) 3,4 is an important physiological response. Resent studies have displayed that IHT inhibits the free radical production, which gives harmful effect to the cells and tissues, and raise the metabolic rate as a result of sympathetic nervous system activation 5,6. Rats trained to intermittent normobaric hypoxia developed an increase of the glycogen contents in the heart and liver parenchymatous cells and offered as many beneficial effects in protecting against myocardial injuries 7,8. Antixidant enzymes and stress proteins may be part of the mechanisms contributing to the cardioprotection of the intermittent hypoxic adaptation 7.Heat shock causes intracellular expression of a specific group of proteins called heat steins (HSPs) that have broad cytoprotective properties 9,10. The first demonstration of HSP- mediated cytoprotection involved the phenomenon of thermotolerance, whereby a brief heat shock conferred protectionagainst subsequent exposure to otherwise lethal hyperthermia 11. Subsequent studies demonstrated that induction of HSPs also protected cells and whole organs against nonthermal cytotoxic agents such as oxidants, nitric oxide, tumor necrosis factor-α, and endotoxin 12-14. Previous studies also demonstrated that induction of HSPs protected against in vitro and in vivo models of acute lung injury and may have therapeutic value for attenuating acute lung injury . HSP 70 has been shown to be protective following ischemic injury. Similarly, a member of the small heat shock family, HSP27 has been shown to play a role in cellular repair and mechanisms of protection against cell stress. In this study, we are used monoclonal antibody to heat shock protein 70 to investigate whether IHT induces the expression of HSP70 in lungs.
Materials and methods
1. Animals
A total of 18 male Wistar rats, aged 12 weeks were used: three animals for control and rest for the experimental groups. They were allowed free access to food and water during intermittent hypoxic training.
2.Intermittent hypoxic training:
Hypoxia was induced by exposure to 10% oxygen with machine. The rats were sustained hypoxia for 15min one time a day for 3, 7, or 21 consecutive days, respectively. Control rats exposured toroom air.
3. Light and electron microscopy.
At 3D, 7D and 21 Days after intermittent hypoxic training (IHT), the animals were anesthetized with pentobarbital sodium (50mg/kg) and lungs were removed. For light microscopic examination, the tissues were fixed with 10% formalin in phosphate buffer solution, and embedded in paraffin.
Then paraffin sections of 2 μm in thickness were prepared and stained with hematoxylin and eosin. For electron microscopic examination, rat lung specimens were cut into 2mm3 blocks, fixed 2.5% glutaraldehyde in 0.1M phosphate buffer and
postfixed with 1% osmium tetroxide, dehydrated in a graded alcohol series and embedded in Epon 812. Semithin sections stained with toluidine blue were used for high light microscopy and selection of areas for thin sectioning. Thin sections were cut with 5000 Ultrotome, stained with uranyl acetate and lead citrate. The sections were examined under a JEM- 1010 transmission electron microscope.
4. Immunohistochemistry
The Streptavidin Biotin Peroxidase Complex (SABC) Method was employed for immunohistochemistry for heat shock protein (HSF70). Briefly, deparaffinized sections were treated for 30 min with 0.3% H2O2 to block endogenous peroxidase and rinsed again 3×5 min inPBS. Sections were then incubated for 30 min with block nonspecific reactive sites by applying 1 :20 normal goat serum and incubated with Monoclonal anti-HSP antibody (NOVO) diluted 1:40 in PBS for 60 min. After washing in PBS, they were incubated with goat anti-mouse immunoglobulins for 10 min and then incubated with a mixture of streptavidin and biotinylated horseradish peroxidase for 5 min. After washing in PBS, reacted with 0.05% 3 – 3′ diaminobenzidine (DAB) containing 0.01 %H2O2.
Results
Light microscopic observations
The morphological observations in the control group were normal from 3 days to 21 days. After three days IHT, the remarkable interstitial edema,increased thickness of the alveolar septa, marked capillary dilatation,proliferation of interstitial cells, the collapse of alveoli and dilation of the alveolar ducts were observed compared with controls (Fig. 1A). After 7 days IHT, those pathologic changes decreased than 3 days (Fig.1B). After 21 days of IHT, the nearly normal structure of lungs was observed (Fig.1C).
Immunohistochemical observation
The heat shock protein 70 monoclonal antibody was used to stain the lung tissue from the control and IHT rats immunohistochemically. In control lungs, there are weak positive staining for HSP70 observed in the bronchial epithelial cells as well as in some alveolar type II epithelial cells.
After 3 days IHT, HSP70 was moderately expressed in alveolar type I ,type II epithelial cells, macrophages and bronchial epithelial cells. HSP70 expressed in cytoplasm and nuclei (Fig.2A). After 7 days IHT, expression of HSP70 was same as 3 days IHT (Fig.2B). After 21 days IHT, there was strong septa also were displayed. By 7 days, the congestion of capillary, type II cell proliferation, and lipid drops in alveolar septa were observed. By 21 days,congestion of capillary, increased capillaryendothelial volume, lipid drops in the alveolar septa and proliferation of alveolar type II cells were noted(Fig.3B).
Discussion :
The principle of intermittent hypoxic training (IHT), with repeated short-term inhalation of hypoxic mixtures had been proposed by S. Strelkov and his associates in the early of 1980’s based on their obstetrical practice. Intermittent hypoxic training (IHT) has showed promise for prevention and treatment of some diseases and efficiently produces great advancement in athletic training 1,2. The mechanism of IHT remains unidentified. A number of mechanisms have been postulated including optimizing both hypothalamic-pituitary-adrenal axis functioning and free radical-mediated process control 5, increase of the quantity and secretory activity of peptidergic neurons of the paraventricular hypothalamic nucleus (PHN) 16,enhancement of neurotransmission in the carotid body (CB) as well as in central
structures through NADPH oxidase stimulation 17, increase in ventilatory response under repetitive hypoxia, changes in suprapontine facilitation of resporatory activity and Changes in monoamine metabolism or release 18, and raise the metabolic rate as a result of sympathetic nervous system activation 6.
In our clinical practice, we found that IHT could release the stress in some patients. In this study, we found that IHT induced the expression of HSP70. The response of cells or organisms to stress such as exposure to heat or chemicals is associated with the induction of heat shock proteins (HSPs). Heat shock proteins (HSPs) are an evolutionarily conserved group of proteins that are highly inducible by a wide variety of stressors. HSPs are grouped by molecular weight and amino acid sequence similarity into five main families: The high molecular weight 100-110kDa family; the 83-90kDa family; the 70kDa family ranging from 66 to 78kDa and containing the highly inducible HSP 70; the 60kDa family present in bacteria,mitochondria, and chloroplasts; and a diverse group of small HSPs ranging from 15 to 30kDa. Of great interest are observations demonstrating that once a heat shock response has been induced, the cells or organs can show remarkable resistance to subsequent metabolic stress. Heat shock protein 70 (HSP70) has been shown to have a protective role in ischemic disease,inflammation, infection and a potential role in antigen processing as well as a possible regulatory role in cytokine biosythesis 13,14. HSP70 exists in the cell in equilibrium between its free state, in the cytoplasm, and its bound state, protecting proteins in the nucleolus, perhaps either by helping refold some of the unfolded ribosomal proteins or by solubilising the denatured ribosomal proteins to facilitate their turnover. During release from heat shock and as the nucleoli begin to recover their normal activities, most of the HSP 70 returns to the cytoplasm. Stress proteins have an important role in normal cellular physiology apart from participation in the stress response. Under normal conditions, stress proteins are involved in the successful folding, assembly, intracellular localization, and secretion of nascent protein chains as they emerge from the ribosome. Stress proteins also function to regulate the degradation of proteins to prevent the accumulation of protein aggregates within the cell. Cultured bovien and ovine pulmonory artery endothelial cells and guinea pig airway epithelial cells and alveolary macrophages expressed abundant HSP 70 after thermal stress14, 19, 20. In rabbit alveolar type II cells, the process of cell isolation itself induced stress protein expression 21. In vivo thermal stress increased stress protein expression in the lungs and other organs of rats, and the time courses and relative magnitudes of expression differed among organs 22,23. Bonay et al demonstrated limited stress protein expression in normal human lungs. HSP90, HSP 70 and HSP63 were selectively expressed in proximal bronchiolar epithelium and alveolar macrophage. In contrast, more distal bronchiolar epithelium, type I and type II alveolar cells, and stromal cells did not express stress proteins 24. HSP 70 expression was substantially increased in airway epithelium and alveolar macrophages of patients with asthma compared with control subjects 25. In this study, we observed the morphologic changes of lungs and expression of HSP70 after rat IHT. We found that that pulmonary damage occurred at 3 days IHT, one week IHT later, the pulmonary damage repaired in hypoxic animals. The expression of HSP70 observed in alveolar type I, type IIepithelial cells,macrophages and bronchial epithelial cells after 3 dr 3 days IHT, andcontinually expressed until 2l days IHT. Expression of HSP 70 in 21 days IHT was stronger than 3 days IHT. This revealed that IHT could induce HSP70 in alveolar type I , type II epithelial cells, macrophages and bronchial epithelial cellsveolar type I, type II epithelial cells, macrophages and bronchial epithelial cells after IHT. HSP70 expressed after 3 days IHT, and continued to 21 days IHT. Although the exact significance of these data is still unresolved, it is proposed expression of HSP may be a mechanism of adaptation of hypoxia with IHT.
Reference
1 Serebrovskaya T, Swanson R, Karaban IN, Serebrovskaya Z, Kolesnikova EE. Intermittent hypoxia alters hypoxic ventilatory responses. Fiziol Zh. 45(5): 9-18, l999
2 Serebrovskaya T, Swarotective effect of stress protein induction in a rat model of acute lung injury caused by intratracheal administration of phospholipase A1 and systemic administration of endotoxin 22,23.This study is the first to demonstrate an expression of HSP 70 in abbins PA.Alterations in respiratory control during 8 h of isocapnic and poikilocapnic hypoxia in humans. J Appl Physiol. 78: 1098-107,1995
4 Schoene RB, Roach RC, Hackett PH, Sutton JR, Cymerman A, Houston CS, Operation Everest II: ventilatory adaptation during gradual decompression to extreme altitude. Med Sci Sports & Exercise. 22:804- 10,1990
5 Adiiatulin AI, Piliavskaia AN, Takchuk EN, Guliaeva NV, [Various mechanisms of protective action of interval hypoxic training during preparation for abdominal dapy. Adaptation Biology and Medicine(Vol.3).
6 Cao KY, Zwillich CW, Berthon-Jones M, Sullivan CE. Increased normoxic ventilation induced by repetitive hypoxia inconscious dogs. J Appl Physiol. 73:2083.8, l992
7. Zhuang J, Zhou Z. Protective effects of intermittent hypoxic adaptation on
myocardium and its mechanisms. Biol Sinals Recept. 8:316-22,1999.Review.
8. Lebkova NP, Chizhov AI, Bobkov II. The adaptational intracellular mechanism regulating energy homeostasis during intermittent normobaric hypoxia. Ross Fiziol Zh lm I M Sechenova. 85:403-11, 1999
9. Minowada G, Welch WJ: Clinical implications of the stress response. J Clin. Invest. 95:3-12, 1995
10. Wang HR, Eispe JR: The stress response and the lung. Am J Physiol 273: L1-9, l997
11. Gerner EW, Schneider MJ: Induced thermal resistance in Hela cells. Nature 256:500-2, 1975
12. Meerson FZ, Malyshev I Yu, Zamotrinsky AV. Differences in adaptive stabilization of structures in response to stress and hypoxia relate with the accumulation of hsp 70 isoforms. Mol Cell Biochem. 111:87-95, 1992
13. Koh Y, Lim CM, Kim MJ, Shin TS, Lee SD, Kim WS, Kim DS, Kim WD. Heat shock response decrease endotoxin-induced acute lung injury in rats. Respirology.4:325-30, 1999
14. Wong HR, R.J Mannix, J.M. Rusnak, A. Boota, H. Zar, S. C. Watkins, J.S. Lazo,B.R. Pitt. The heat shock response attenuates lipopolysaccharide-mediated apoptosis in cultured sheep pulmonary artery endothelial cells. Am. J. Respir. Cell Mol. Biol. :745-51, 1996
15. Wong HR, Ryan M, Menedez IY, Denenberg A and Wispe JRlar hypothalamic nucleus and brain stem neurons in rats]. [Russian] Ross Fiziol Zh lm I. M. Sechenova. 84(3): 173-81, 1998
17 0lano M, Song D, Murphy S, Wilson DF, Pastuszko A. Relationships of dopamine, cortical oxygen pressure, and hydroxyl radicals in brain of newborn piglets during hypoxia and posthypoxic recovery. J Neurochem. 65(3):1205-12, 1995
18. Soto-Arape I, Burton MD, Kazemi H. Central amino acid neurotransmitters and the hypoxic ventilatory response. American J Respir Crit Care Med. 151(4):1113-20, 1995
19. Rinaldo J. E, M. Gorry R, Stricter H, Cowan R, Abdolrasulnia V. Shepard. Effect of endotoxin-induced cell injury on 70-kD heat shock proteins in bovine lung endothelial cells. Am. J. Respir. Cell Mol. Biol. 3:207-16, 1990
20. Cohen D.S, E. Palmer WJ, Welch, D. Sheppard. The respose of guinea pig airway epithelial cells and alveolar macrophages to environmental stress. Am. J. Respir. Cell Mol. Biol. 5:133-43, 1991
21. Brandes M. E, J. N Finkelstein. Induction of the stress response by isolation of rabbit type II pneumocytes. Exp. Lung.Res. 15: 93-111, 1989.
22. Villar J, J. D. Edelson, M. Post, B. Mullen, A. S. Slutsky. Induction of heat Stress proteins is associated with decreased mortality in an animal model of acute lung injury. Am. Rev. Respir. Dis. l47:177-81, l993.
23. Villar J.,S.P.Ribeiro,J.B.M.Mullen,M.Kuliszewski,M.Post,A.S.Slutsky.Induction of the heat shock response reduces mortality rate and organ damage in a sepsis-induced acute lung injury model. Crit. Care Med. 22:9 B ± through preventing I o B kinase activation in 0respiratory epithelial cells. J. Immunol 164:541 6-23, 2000
27. Yoo, C.G., Lee S., Lee C. T., Rim Y. W., Ham S. K., and Shin Y. S. Anti-inflammatory effect of heat shock protein induction is related to stabilization of I κ B α through preventing I κ B kinase activation in respiratory epithelial cells. J. Immunol 164:5416-23, 2000
28. Simon M. M, A. Reikerstolfer, A. Schwarz, C. Kronis, T. G.Lunger, M. Jaatela, T. Schwarz. Heat shock protein 70 overexpression affects the response to ultraviolet light in murine fibroblast. J. Clin. Invest. 95:926-933,1995
Figure Legends
Fig 1 Change of light microscope in IHT rat lungs. A: 3 days IHT. B:7days IHT.
C: 21 days IHT.
Fig 2 Immunohistochemical staining of rat lung with anti-HSP70 after IHT.
A: 3 days IHT. B: 7 days IHT. C: 21 days IHT.
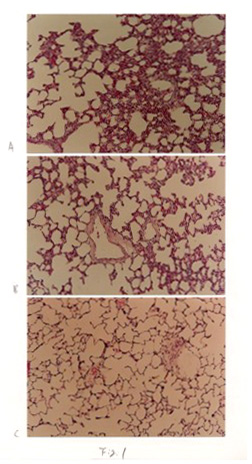
Fig 3 Ultrastructural changes of lung in IHT: A: 3 days IHT. B: 21 days IHT